英华教育(青岛)语言中心--SAT化学练习:Equilibrium and Reaction Rates-英华外语整理SAT化学练习,供学员学习。班制灵活,随报随学,英华教育咨询热线:400-0532-757
Use the following diagram to answer questions 1–3:
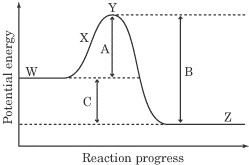
1. Which letter corresponds to the activation energy of the reaction?
(A)A
(B)B
(C)C
(D)Y
(E)X
2. Which letter corresponds to the change in energy for the overall reaction?
(A)A
(B)B
(C)C
(D)Y
(E)X
Statement IStatement II
3.The reaction shown above is exothermic. BECAUSE Energy difference B is greater than energy difference A.
4.A system is at equilibrium when the rate of the forward reaction is equal to the rate of the reverse reactions. BECAUSE At equilibrium, the concentration of the products is equal to that of the reactants.
5. Which of the following statements best describes the condition(s) needed for a successful formation of a product in a chemical reaction?
(A)The collision must involve a sufficient amount of energy, provided from the motion of the particles, to overcome the activation energy.
(B)The relative orientation of the particles has little or no effect on the formation of the product.
(C)The relative orientation of the particles has an effect only if the kinetic energy of the particles is below some minimum value.
(D)The relative orientation of the particles must allow for formation of the new bonds in the product.
(E)The energy of the incoming particles must be above a certain minimum value and the relative orientation of the particles must allow for formation of new bonds in the product.
6. The catalyzed pathway in a reaction mechanism has a _____ activation energy and thus causes a _____ reaction rate.
(A)higher, lower
(B)higher, higher
(C)lower, higher
(D)lower, steady
(E)higher, steady
7. Write the equilibrium expression for the following reaction:
(A)[A]2[B][D]
(B)
(C)
(D)
(E)
8. If at a given temperature the equilibrium constant for the reactionis Kp, the equilibrium constant for the reactioncan be represented as
(A)
(B)
(C)
(D)
(E)
9. The value of the equilibrium constant, K, is dependent on
I. The temperature of the system
II. The concentration of the reactants
III. The concentration of the products
IV. The nature of the reactants and products
(A)I, II
(B)II, III
(C)III, IV
(D)I and IV
(E)I, II, and IV
10. Consider the system below at equilibrium. Which of the following changes will shift the equilibrium to the right?
I. Increasing the temperature
II. Decreasing the temperature
III. Increasing the pressure on the system
(A)I only
(B)II only
(C)III only
(D)I and III
(E)II and III
Explanations
1. A
The activation energy is the energy that must be overcome for the reaction to proceed. Also remember that for a reaction to occur, the collisions between molecules must be sufficiently energetic and of the proper geometric orientation.
2. C
The energy change for the overall reaction is simply the difference between the energies of the products and reactants, and this is indicated by the letter C on the diagram.
3. T, T
(Fill in CE.) The energy change indicated by A on the diagram represents the activation energy of the reaction—the energy investment required to form the activated complex Y, also known as the energy that must be put into the system to make the reaction go. B on the diagram represents the energy released when the unstable transition state molecule Y goes to a lower energy state as the products Z. The reaction is exothermic when the energy payoff exceeds the energy investment, and since the second statement is the reason for the first statement, you would fill in the CE oval.
4. T, F
(Do not fill in CE oval.) The first statement is true—when a chemical reaction is at equilibrium, the rate of the forward reaction is equal to the rate of the reverse reaction, in which the reactants are formed. However, statement II is incorrect and is a common misconception. The amount of reactant and product remain constant at equilibrium but usually do not equal each other. Since the second statement is false, you would not fill in the CE oval.
5. E
Two conditions must be met in order for a chemical reaction to occur. First of all, the molecules must collide with sufficient energy, and second, the molecules must collide with such an orientation that the product bonds can be formed.
6. C
The addition of a catalyst lowers the activation energy, thus speeding up a chemical reaction.
7. D
First, be on the lookout for pure liquids and pure solids: These do not appear in equilibrium constant expressions. C is a solid, so do not include it in the expression. Second, remember that the expression is written as the product of the products raised to the power of their coefficients over the product of the reactants raised to the power of their coefficients. Thus, the correct answer is
8. C
The reaction is reversed, so take the reciprocal of Kp, and the coefficients in the balanced equation are halved, so you’ll raise Kp to the power, which is the same as finding the square root of Kp. The correct answer is
9. D
Look through the statements carefully, one by one. First, you know that the value of K depends on the temperature of the system: if you change the temperature, the value of K changes, so item I is correct. Next, K is independent of the concentrations of the reactants or products, so both items II and III are incorrect. K is dependent on the nature of the products and reactants, however, so IV is correct.
10. E
This question combines two concepts. The reaction is exothermic, so think of heat as a product. Increasing the temperature has the same effect as increasing a product’s concentration, so it causes a shift to the left, meaning statement I cannot be in the answer choice. Decreasing the temperature (removing heat) would have the same effect as removing a product (since the reaction is exothermic), so this would cause a shift to the right, and II must be in the correct answer choice. Finally, since all reactants and products are in the gas phase, and there is a total of four moles of gas on the left and a total of two moles of gas on the right, increasing the pressure will push the reaction toward the side with the fewest moles of gas. In this case, the side with the fewer moles of gas is the products side, so this also causes a shift to the right. III is also correct, and answer choice E is correct.













 (高老师)
(高老师)


